I know, I just did a ‘Too Cool’ post, but then I came across this and it certainly deserves to be in here. Neatorama linked me over to an article on Quartz about a rather intriguing accomplishment in macro work, which is that little purple dot in the center of the image below, because this is apparently a single atom, captured with a conventional camera as well.
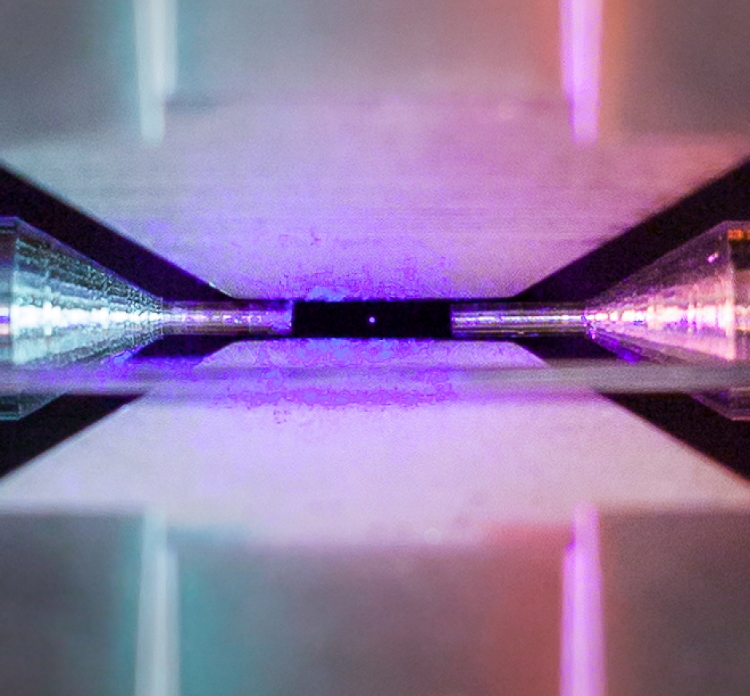
Really, check out the article, because it tells the details, but just so you know, the gap between the two electrodes flanking that dot on the sides is 2mm, slightly less than the shaft of a cotton swab.
Now, if you’re thinking like I did that this seems a little huge for an atom, which defeats even electron microscopes, well, here’s the deal as I understand it (you know, quantum physicist me.) It took a certain wavelength of laser to illuminate the atom, as well as a long exposure. Atoms are too small to reflect light, or more specifically, for individual photons to bounce off of because they’re little more than photons in size in the first place, and the energy of the photon typically affects the atom. It’s part of the whole quantum indeterminacy thing, because just trying to figure out where an atom is in the first place is virtually guaranteed to alter or move it. In this case, it seems that the strontium atom was absorbing photons into its structure temporarily, essentially converting the photon into energy along the electron shells/orbits/paths, which would bump the electron into a higher shell/orbit. Shortly, the electron drops back into its original position in regards to the nucleus, re-emitting that energy again as a photon, and that’s what the camera was capturing. Only, a single photon isn’t enough to register much on the digital sensor, so it had to keep happening over time, which is why it took a longer exposure. What you’re seeing isn’t an atom per se, but a collection of emitted photons, which may well not be as pinpoint precise as we’d like. There also remains the chance that the effects of the atom curve the photon paths a little, and/or that the ion trap holding it suspended can’t keep it perfectly still, but that’s just me speculating.
This is why science is so damned cool. We’ve known for decades that atoms are too small for photons (“visible light”) to reflect from, and even very small details take an electron microscope, because electrons don’t have wavelengths and don’t need focusing as such. But this guy just thought, Fine, I’ll make it glow on its own, and there you have it. Slick.




















































